There are two primary energy sources for the body:
·
Carbohydrates in the form of sugars (i.e.
glucose) produce energy by glycolysis to make acetyl CoA for the citric acid
cycle.
·
Fats in the form of triglycerides
produce energy by beta-oxidation to make acetyl CoA for the citric acid cycle.
Carbohydrates
and fats are converted to energy in cellular organelles called mitochondria.
Energy is produced by both glycolysis and the citric acid cycle. Environmental toxins can inhibit the
production of energy from both carbohydrates and stored fats resulting in
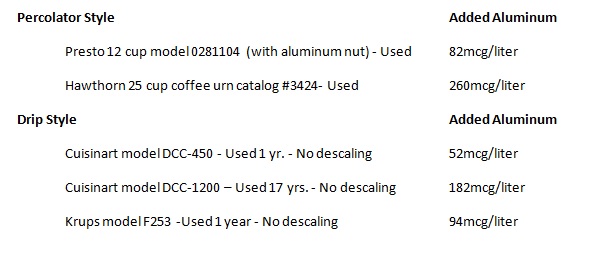
obesity. For instance aluminum at a concentration of some drinking water in the
U.S. inhibits glycolysis.
Since development
of the Bayer process for aluminum purification from bauxite in 1888, there has
been a steady increase in the amount of aluminum humans ingest and accumulate. Aluminum, at levels found in some drinking
water (108ppb,108mcg/liter, 4mcM), inhibits hexokinase, an enzyme that
catalyzes the first step in carbohydrate
metabolism (i.e. glycolysis)1. The biochemical response to the inhibition of
glycolysis is the conversion of carbohydrates to fat as triglycerides comprised
of long chain fatty acids2.
This fat can be stored in adipose tissue or metabolized for energy. However, aluminum also inhibits the
production of L-carnitine required for movement of long chain fatty acids in
stored fat to the mitochondria for conversion to energy3-6. Therefore aluminum inhibits two key steps in
metabolizing carbohydrates and fats for energy generation:
·
Aluminum inhibits the first
step of carbohydrate metabolism called glycolysis1. Inhibition of
glycolysis promotes the conversion of carbohydrates to stored fats (e.g.
lipogenesis)2.
·
Aluminum inhibits the
biosynthesis of L-carnitine3-6. L-carnitine is required for mobilizing
stored fat as long chain triglycerides for mitochondrial energy production7.
The
result of aluminum ingestion is therefore, more fat from carbohydrate, more fat
being stored, and less fat being utilized for energy, resulting in obesity that
does not respond to dieting.
Ketogenic Diet of Medium Chain Triglycerides for Coping with
Aluminum Toxicity
·
Long chain essential fatty
acids (e.g. linoleic and alpha-linolenic acids)
·
Long chain non-essential
fatty acids (i.e. EPA and 22C DHA)
·
Medium chain fatty acids (i.e. lauric acid found as 50% of coconut oil)
Moderate
Carbohydrate Diet: 30% fat - 40%
carbohydrate - 30%
protein
Ketogenic
Diet: 60% fat - 5% carbohydrate - 35% protein
After
eight weeks the moderate carbohydrate dieters lost more weight than the ketogenic
dieters. The researchers concluded that
the ketogenic diet did not offer any significant metabolic advantage over the moderate
carbohydrate diet12.
There
are supplements of biochemicals naturally found in your body that taken daily
will result in improved stored fat utilization and weight loss. These supplements are:
·
Dissolved silica (a.k.a. OSA)
for lowering your body-burden of aluminum13-15
·
CoQ10 for improving your
energy and cognition16
·
PQQ for increasing
mitochondrial biogenesis and cognition16-18
·
EPA (eicosapentaenoic acid) for
reducing triglycerides by 5 to 10%19
·
PA (palmitoleic acid) for
reducing triglycerides by 15% and LDL by 8%20
·
Vitamin D for reducing
triglycerides by 23%21
Lowering
triglycerides and LDL decreases the risk of vascular disease, heart attack, and
stroke. For more details on these
supplements see my book “Prevent Alzheimer’s, Autism, and Stroke”22.
Ketogenic Diet with Fat from Medium Chain
Triglycerides
Medium
chain triglycerides (MCT), as opposed to long chain (i.e. 18 carbon atoms)
triglycerides (LCT), do not require L-carnitine for mobilization and conversion
into energy by the mitochondria10.
Therefore the metabolism of MCT is not inhibited by aluminum. Also the
oxidative utilization (sum of digestion, absorption, and oxidation) of MCT can
be 3 to 4 times greater than for LCT10. These results were obtained with animals
preconditioned to survive, like Lieutenant Schwatka, on a ketogenic diet10.
Therefore
the modern equivalent of the “Schwatka Imperative” is to either:
·
Remain obese while surviving
on a diet of medium chain triglycerides or
·
Lose some weight by
decreasing aluminum accumulation and eating a moderate carbohydrate diet.
Many
people are opting for the MCT diet, such as coconut oil, without lowering
aluminum. This will provide more energy
and improved cognition. Unfortunately it will not result in weight loss since
aluminum is still inhibiting the mobilization and conversion of stored long
chain fatty acids to energy. Also:
·
MCT or Coconut oil does not
contain essential fatty acids (e.g. linoleic and alpha-linolenic acid)
·
Lauric acid, comprising 50%
of coconut oil, increases LDL by 16% in humans and LDL is linked to vascular
disease, such as stroke and heart attack11
References
1. Lai,
J.C., and Blass, J.P.; Inhibition of brain glycolysis by aluminum; J.
Neurochem.; Feb.; 42(2):438-46 (1984)
2.
Mailloux, R.J., et al.; Hepatic response to aluminum toxicity: Dsylipidemia and
liver diseases; Exper. Cell Res.; 317:2231-2238 (2011)
3.
Gaballa, I.F., et al.; Dyslipidemia and disruption of L-carnitine in aluminm
exposed workers; Egyptian J. Occup. Med.; 37(1):33-46 (2013)
4.
Lemire, J., et al.; The disruption of L-carnitine metabolism by aluminum
toxicity and oxidative stress promotes dyslipemia in human astrocytes and
hepatic cells; Toxicol. Lett.; Jun.; 203(3):219-26 (2011)
5. Waly,
M. I-A., et al.; Activation of methionine synthase by insulin-like growth
factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal;
Mol. Psychiatry; 9:358-70 (2004)
6. Waly,
M. I-A., and Deth, R.; Neurodevelopmental toxins deplete glutathione and
inhibit folate and vitamin B12-dependent methionine synthase activity – a link
between oxidative stress and autism, FASEB J.; 22:894 1 (2008)
7.
Fritz, I.B., Kaplan, E., Yue, K.T.; Specificity of carnitine action on fatty
acid oxidation by heart muscle; Am. J. Physiol.; Jan.; 202:117-21 (1962)
8.
Schwatka, F.; The Long Arctic Search; Stackpole, E.A., Editor; No. 44; The
Marine Historical Association, Inc.; Mystic, CT (1965)
9.
Beattie, O., and Geiger, J.; Frozen in time – The fate of the Franklin
Expedition; Bloomsbury (2004)
10. Heo,
K.N., et al.; Medium-chain fatty acids but not L-carnitine accelerate the
kinetics of [14C]triacylglycerol utilization by colostrum-deprived newborn
pigs; J. Nutr.; 132:1989-1994 (2002)
11.
Tsai, Y.H., et al.; Mechanisms mediating lipoprotein responses to diets with
medium chain triglyceride and lauric acid; Lipids; Sep.; 34(9):895-905 (1999)
12.
Johnston, C.S., et al.; Ketogenic low-carbohydrate diets have no metabolic
advantage over nonketogenic low-carbohydrate diets; Am. J. Clin. Nutr.;
83:1055-61 (2006)
13.
Edwardson, J.A., et al.; Effect of silicon on gastrointestinal absorption of
aluminum; The Lancet; 342(8865):211-12 (1993)
14.
Carlisle, E.M., and Curran, M.J.; Effect of dietary silicon and aluminum on
silicon and aluminum levels in rat brain; Alzheimer Dis. Assoc. Disord.;
1(2):423-30 (2013)
15.
Davenward, S,, et al.; Silicon-rich mineral water as a non-invasive test of the
'aluminum hypothesis' in Alzheimers disease; J. Alzheimer's Dis.; 33(2):423-30
(2013)
16.
Nakani, M., et al.; Effect of pyrroloquinoline quinone (PQQ) on mental status
of middle-aged and elderly persons; Food Style; 21 13(7):50-3 (2009)
17.
Chowanadisai, W., et al.; Pyrroloquinoline quinone stimulates mitochondrial
biogenesis through cAMP response element-binding protein phosphorylation and
increased PGC-1 alpha expression; J. Biol. Chem.; Jan.; 285(1):142-52 (2010)
18.
Onyango, I.G., et al.; Regulation of neuron mitochondrial biogenesis and
relevance to brain heath; Biochim Biophys Acta; jan.; 1802(1):228-34 (2010)
19.
Bernstein, A.M., et al.; Purified palmitoleic acid for the reduction of
high-sensitivity C-reactive protein and serum lipids: a double blinded, placebo
controlled study; J.Clin. Lipidol.; 8(6):612-7 (2014)
20.
Harris, W.S.; n-3 Fatty acids and serum lipoproteins: human studies; A. J.
Clin. Nutr.; 65(suppl.):1645S-54S (1997)
21.
Rejnmark, L., et al.; Simvastatin does not affect vitamin D status, but low
vitamin D levels are associated with dyslipidemia; Results from a randomized,
contolled trial: Internat. J. Endrocrin.; Article ID 957174 (2010)
22.
Crouse, D.N.; Prevent Alzheimer's, autism, and stroke, with 7 supplements, 7
life-style choices, and a dissolved mineral; Etiological Publishing (2016)