Before purchasing a coffee maker write to the company and ask if the water comes in contact with aluminum. Unfortunately manufactures change their manufacturing.
My 91 year old Mom has Alzheimer’s disease. In an effort to slow and possibly reverse the
decline of her short term memory and the progression of her Alzheimer’s disease,
I have been helping Mom lower her ingestion of ionic aluminum. Aluminum ions are neurotoxic, killing neurons
and inhibiting the ability of neural networks in the brain to store memories.
The 7 largest epidemiology studies of aluminum in drinking
water concluded that there is a greater risk of Alzheimer’s disease when routinely
drinking water or any beverage with greater than 100mcg per liter of ionic
aluminum. This data convinced the World Health Organization to set a 100mcg
per liter maximum limit of aluminum in drinking water.
Before age 77 people with Alzheimer’s accumulate aluminum
faster than normal, partially accounting for why some of them have early onset Alzheimer’s. But all people older than 77 accumulate
aluminum faster than normal accounting for why in the U.S. one in three people over
80 have Alzheimer’s. Therefore it is
preventative to remove daily sources of aluminum ions from the diet and
drinking water of everyone and particularly the elderly.
Surprisingly one of mom’s daily sources of aluminum is her Black
& Decker drip style coffee maker (Model 1050). After removing the safety screws on the
bottom of the coffee maker, I found a heated horseshoe shaped aluminum tube that
heats all the water that flows from the reservoir to the carafe. In the process of heating the water, the inside
of the hot metallic aluminum corrodes adding neurotoxic aluminum ions to the
resulting coffee.
Bottom view of Black
& Decker Model 1050W With Bottom Plate Removed
In order to find how
much aluminum was added to mom’s coffee by the Model 1050 coffee maker, I
tested and compared the aluminum concentration in water both before being added
to the coffee maker and after being heated and passed into the carafe. I found
the amount of aluminum added by Mom’s coffee maker to be extremely disturbing because
of Mom’s age and condition and because there are millions of these types of
coffee makers being used every day to make aluminum-laced neurotoxic coffee.
As the coffee maker is used, aluminum on the inside of the
tube corrodes by pitting, thereby increasing the rate of corrosion. Also if the coffee maker is used with hard
water, a calcium and magnesium carbonate scale forms on the inside of the heated
aluminum tube. This carbonate scale is
porous and does not slow the rate of aluminum corrosion. The B&D
Model 1050 used for 1 year with hard water makes coffee with an aluminum level of
264% of the World Health Organization’s maximum level in drinking water.
Fiji water contains 94ppm of silica. After a coffee maker is
used with Fiji water for 1 year, a silica scale forms on the inside of the
aluminum tube. Silica scales are harder
and less porous than carbonate scales and reduce the aluminum corrosion rate. Descaling the B&D Model 1050 with 50%
vinegar in water removes both carbonate and silica scales but results in the
descaled coffee maker adding even more aluminum to the coffee.
Due to the high level of aluminum found with the used B&D
Model 1050, my interest in coffee makers became a temporary obsession. With the
help of family and friends I began collecting and testing any coffee maker I
could find. As the word of my initial results spread, discarded coffee makers
began showing up on my doorstep. Shockingly
I discovered that coffee contacts aluminum in almost all my family and friend’s
coffee makers.
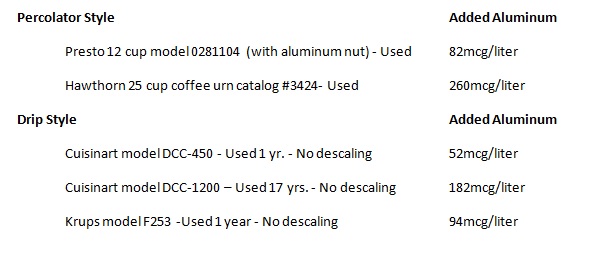
Examples of these coffee makers include the Presto
percolator that is all stainless except for a large aluminum nut that holds the
heater in place. Also the Krups F253 that is advertised with a brass heating
tube but after removing the security screws on the bottom I discovered an
aluminum heating tube.
After finding such shockingly high aluminum levels in used
coffee makers, my goal became trying to find for Mom an electric coffee maker in
which the coffee never contacts aluminum. Just
because the manufacturer calls their product a “stainless steel coffee maker”
does not mean the coffee never contacts aluminum. The Jura Capresso MG900 is the only coffee
maker found so far that states on the box “coffee never contacts aluminum”. After much research on the internet and contacting
coffee maker manufacturers the following coffee makers were obtained, tested,
and found to not add aluminum to coffee:
Any Bunn coffee maker that has an internal stainless steel tank is fine.
Mom used the Krups Moka Brew for several months but ultimately found the carafe to be too hard to unlatch because of her arthritis. Now she uses the BUNN Speed Brew and likes its ease of use. Both Mom and Dad have said the aluminum-free coffee tastes better than the aluminum-laced coffee. This is not surprising as many people do not like the metallic taste of aluminum. What makes me happy is that both Mom and Dad have reduced their aluminum ion ingestion by switching to a coffee maker in which the coffee never contacts aluminum. This was an important step in reversing the decline of Mom’s short term memory.
Mom used the Krups Moka Brew for several months but ultimately found the carafe to be too hard to unlatch because of her arthritis. Now she uses the BUNN Speed Brew and likes its ease of use. Both Mom and Dad have said the aluminum-free coffee tastes better than the aluminum-laced coffee. This is not surprising as many people do not like the metallic taste of aluminum. What makes me happy is that both Mom and Dad have reduced their aluminum ion ingestion by switching to a coffee maker in which the coffee never contacts aluminum. This was an important step in reversing the decline of Mom’s short term memory.
video on coffee makers
Video is made by DennisHere are aluminum free coffee makers:
Updated November 2023
Drip Style :
Krups Mocha brew
Bunn Speed/Velocity Brew
Braun coffee maker model KF7000
Capresso MG 900 Difficult to find as of October 2023. Sometimes they are available at the company's website. Link below. I wrote the company asking if they are continuing to make this model and I did not get a response.
order capresso
Beautiful Coffee Maker - Here is information from the company. Dennis will be testing this coffee maker.
From: "Cookwithbeautiful [mailto:info@cookwithbeautiful.com]
Sent: Tuesday, September 27, 2022 8:52 AM
To: adamsonl
Subject: Re: question about your coffee maker
Hi Laurie,
Thank you for reaching out
The water does not come into contact with aluminum
This is the only coffee maker in the Beautiful line"
Percolator: Faberware FCP 240-A (small), Faberware FCP 412 (large)
COLETTI Bozeman Camping Coffee Pot – Coffee Percolator – Percolator Coffee Pot for Campfire or Stove Top Coffee Making – 9 CUP
Here are aluminum free coffee makers no longer available. It appears Keurig has stopped making aluminum free coffee makers. Nespresso Type C110 (the aluminum cups have been tested and they are safe) - not sure this is available anymore.
Beautiful Coffee Maker - Here is information from the company. Dennis will be testing this coffee maker.
From: "Cookwithbeautiful [mailto:info@cookwithbeautiful.com]
Sent: Tuesday, September 27, 2022 8:52 AM
To: adamsonl
Subject: Re: question about your coffee maker
Hi Laurie,
Thank you for reaching out
The water does not come into contact with aluminum
This is the only coffee maker in the Beautiful line"
Percolator: Faberware FCP 240-A (small), Faberware FCP 412 (large)
COLETTI Bozeman Camping Coffee Pot – Coffee Percolator – Percolator Coffee Pot for Campfire or Stove Top Coffee Making – 9 CUP
Here are aluminum free coffee makers no longer available. It appears Keurig has stopped making aluminum free coffee makers. Nespresso Type C110 (the aluminum cups have been tested and they are safe) - not sure this is available anymore.













 Aluminum is a neurotoxin, that is, a poison to the brain and nervous system. Some experts have long speculated that this metal plays a role in Alzheimer’s disease, and evidence is steadily mounting that it indeed does. Fortunately, there’s also evidence that suggests that a number of natural plant extracts and nutrients can prevent and/or reduce aluminum toxicity in the brain and prevent the progression of memory loss and other cognitive deficits.
Aluminum is a neurotoxin, that is, a poison to the brain and nervous system. Some experts have long speculated that this metal plays a role in Alzheimer’s disease, and evidence is steadily mounting that it indeed does. Fortunately, there’s also evidence that suggests that a number of natural plant extracts and nutrients can prevent and/or reduce aluminum toxicity in the brain and prevent the progression of memory loss and other cognitive deficits.